
Natural products have been a cornerstone of drug discovery and development for over a century
 01
01Natural products have been a cornerstone of drug discovery and development for over a century, delivering a plethora of therapeutic agents that have revolutionized medicine. From the ancient use of willow bark, which led to the development of aspirin, to modern advancements such as the discovery of capsaicin for pain, natural products have consistently demonstrated their value in the pharmaceutical landscape, as we have previously covered in our blog series [REF, REF]. These naturally derived compounds offer a rich source of chemical diversity and biological activity that synthetic compounds struggle to match.
Several studies have attempted to quantify the percentage of natural products (NPs) among approved drugs, with notable contributions from researchers like Newman and Cragg. These scholars, along with their colleagues, have published a series of seminal publications that cover the percentage of NPs over different time spans [1, 2]. Their findings have highlighted that NPs comprise up to 50% of all small molecules approved drugs, underscoring the significant role these compounds play in drug discovery and development. While the precise numbers are subject to debate depending on how a natural product is defined, the overarching conclusion is clear: NPs represent a substantial proportion of the drugs approved for clinical use.
These numbers are particularly impressive given the larger trend within the biopharma industry away from NPs and towards synthetic molecules, cell and gene therapies, and large macromolecules like antibody-drug conjugates. While other drug types are getting a lot of press, NPs and NP-like compounds still represent a major proportion of new drugs every year [3].
Given these numbers, the question naturally arises: why are natural products so prevalent among approved therapeutics? Are NPs “easier” or “better” starting points for drug discovery? One possibility is that many NPs have evolved to interact with biological systems, giving them inherent biological compatibility (e.g., cell membrane permeability) and relevance (e.g., ability to bind protein pockets) that can be harnessed for therapeutic purposes. However, it could also be that NPs have been tested in more clinical trials than synthetic compounds, leading to a bias in the data. In our new article, recently published in the Journal of Natural Products, we delve into these questions and explore factors that make natural products such valuable assets in the pharmaceutical landscape [4].
 02
02The first question we wanted to answer is whether NPs owe their high prevalence among approved drugs simply because they are a more frequent starting point for early stage drug discovery. We found the opposite to be true. We began by investigating all chemical structures from small molecules present in patent literature leveraging SureChEMBL (https://www.surechembl.org/). We categorized each structure as a i) NP, ii) synthetic, or iii) hybrid. Instead of relying on a scientist to manually define each chemical structure as in previous publications [1, 2], we used the NP-likeness score [5]. This score indicates how NP-like or synthetic-like a small molecule is, and allows us to conduct a large-scale categorization based on the definitions observed in previous work [6]. Our results show that NPs and hybrids are under-represented in this earlier drug discovery phase compared to their previously reported prevalence among approved drugs (NPs and hybrids combined correspond to less than 25% chemical structures reported in patents). Furthermore, our analysis also revealed that this pattern did not change over the last decade. This result is consistent with prevailing wisdom of natural product drug discovery as a strategy in the modern pharmaceutical industry. This analysis further suggests that NPs achieve their outsized representation in approved medicines despite being the less common starting material.
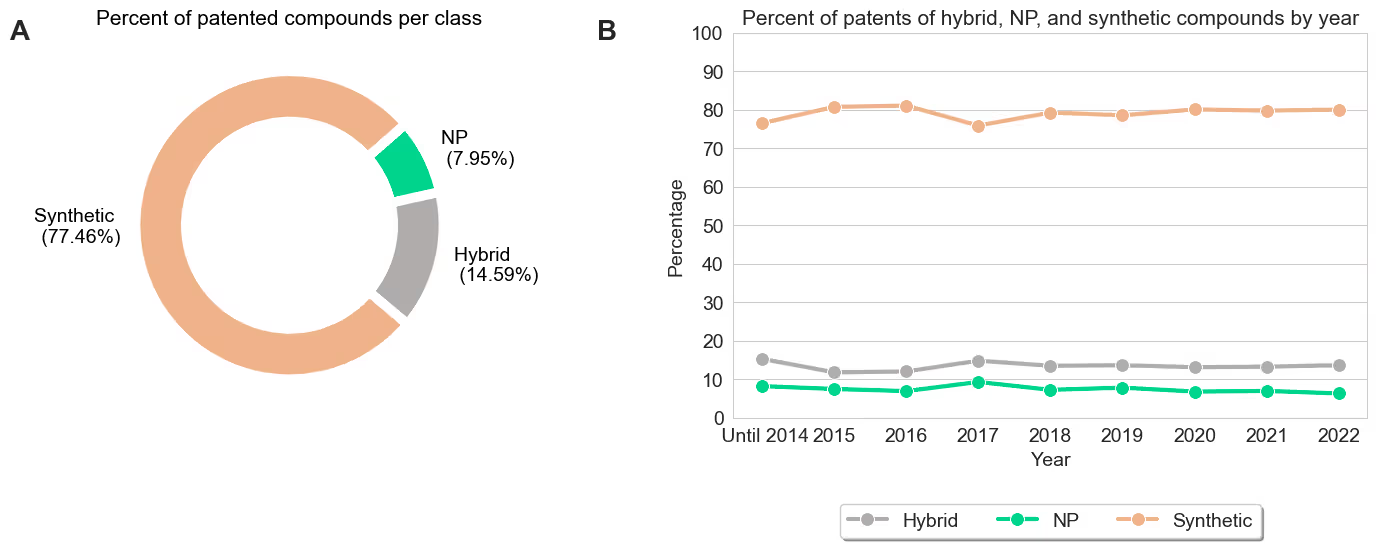
Figure 1. A) Percentage of patent compounds per class (Hybrid, NP, and synthetic). B) Percent of patent applications of each class by year.
 03
03Next, we asked whether this is due to increased chances of success in clinical trials. Because most clinical trials fail, having even a moderate increase in success rates at each phase would result in a major increase in representation amongst approvals.
We systematically harmonized historical clinical trial data from ClinicalTrials.gov and explored the percentage of drugs for each class, stratifying by the latest clinical phase (e.g., phase I, II, III) they were reported in. To complement ClinicalTrials.gov, we leveraged a commercial database of clinical trials (https://clinicalintelligence.citeline.com) and also a set of approved drugs to measure the proportion of NPs among approved drugs.
Figure 2 summarizes our findings and shows how the percentage of synthetic compounds decreases as drugs advance toward the later stages of clinical development while the percentage of both hybrids and NPs increases. Specifically, the proportion of NPs increased from ~20% in phase I to ~26% in phase III, and the proportion of hybrids from ~15% to ~19%. Overall, NPs and hybrids constituted around 45% of compounds in phase III, aligning with proportions observed in approved drugs.
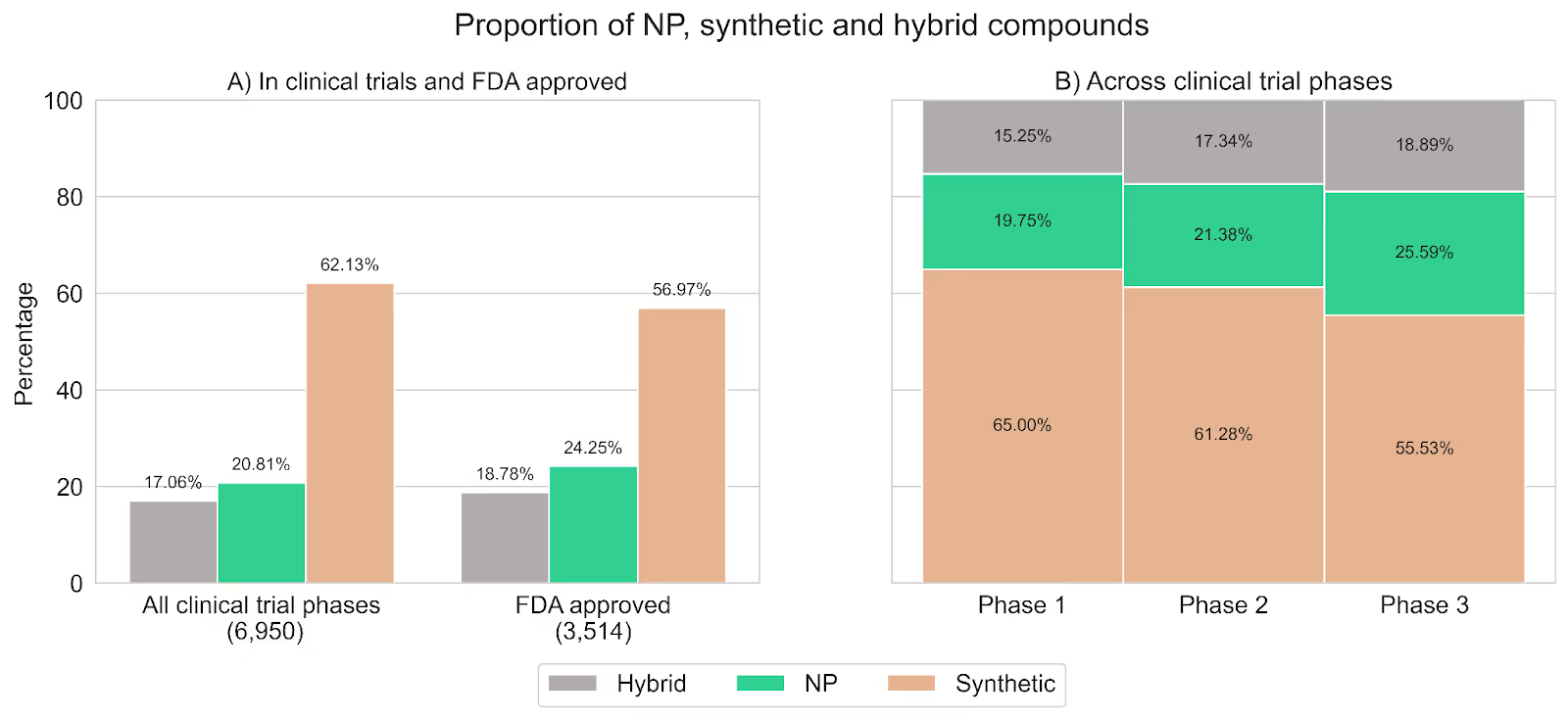
Figure 2. A) Proportion of NP, synthetic, and hybrid compounds in all clinical trials compared to FDA-approved drugs. B) Proportion of NP, synthetic, and hybrid compounds across the phases of clinical trials (data from ClinicalTrials.gov). Compounds are stratified by their latest phase (i.e., phase I/II are categorized as phase II and phase II/III are categorized as phase III).
 04
04We demonstrated that natural products have increased rates of clinical trial success throughout the drug development process. While these studies can’t fully explain why NPs are so successful, the answer may lie in the historical and empirical use of NPs, which provides a wealth of preliminary data supporting their safety and efficacy. The structural diversity and complexity of NPs often lead to better interaction with biological targets, potentially resulting in higher efficacy and fewer adverse effects compared to synthetic compounds.
Because each clinical trial phase is associated with a different endpoint (e.g., phase I is assessing the toxicity of a drug, and Phase II and III its efficacy), we examined the relative changes of each class when progressing from one phase to the next. Unfortunately, organizations are not obliged to disclose the reason for the termination of a trial or reasons for its failure. Thus, it is not possible to address whether NPs are more or less toxic than synthetic compounds. However, as a proxy for this hypothesis, we analyzed public toxicity datasets and investigated differences between in silico toxicity predictions for thousands of NPs and synthetic compounds. We found that natural products tend to be less toxic in a diverse set of toxicity endpoints. Again, our findings cannot be directly extrapolated to clinical trials data, but they show that NPs tend to exhibit less toxicity for the majority of the 32 evaluated endpoints. This is consistent with intrinsic biological relevance and compatibility with biological systems. Similarly to toxicity considerations, efficacy attribution is also hindered by the lack of complete annotations for compounds that did not progress to FDA approval. d. However, one hypothesis that could explain the observed trend is that many NPs have ethnobotany priors (i.e., have been used to treat certain indications by specific cultures) and knowledge of their historical use likely provided clues to their safety and efficacy, giving NPs a headstart in the drug development process.
 05
05Natural products, advantaged by centuries of historical use and diverse chemical structures honed by billions of years of convergent evolution, offer a rich repository of chemical compounds. In our analyses, we found that NPs have an increased likelihood of success in the clinic. Although fewer NPs initially enter clinical trials compared to synthetic compounds, a substantial proportion go on to become FDA-approved drugs. These findings underscore the importance of leveraging natural products in drug development, and in doing so, increase the chances of discovering safer and more effective medicines.
 06
06